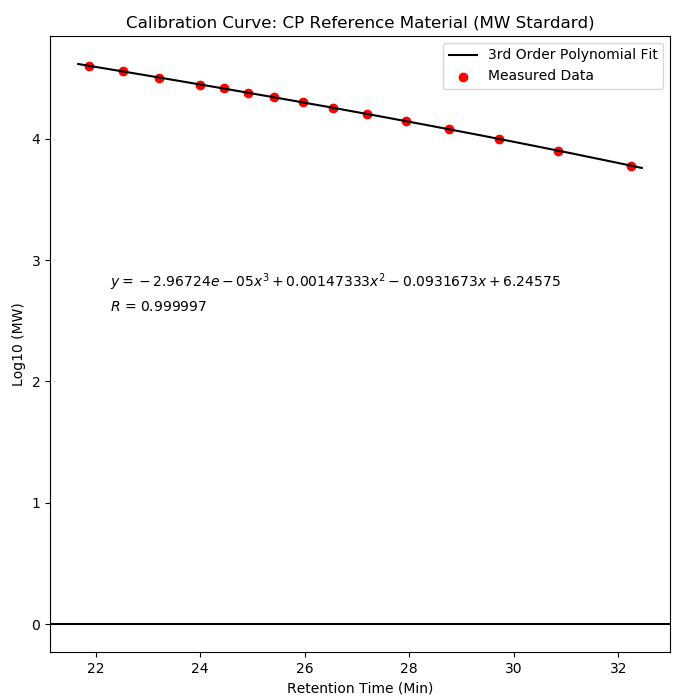
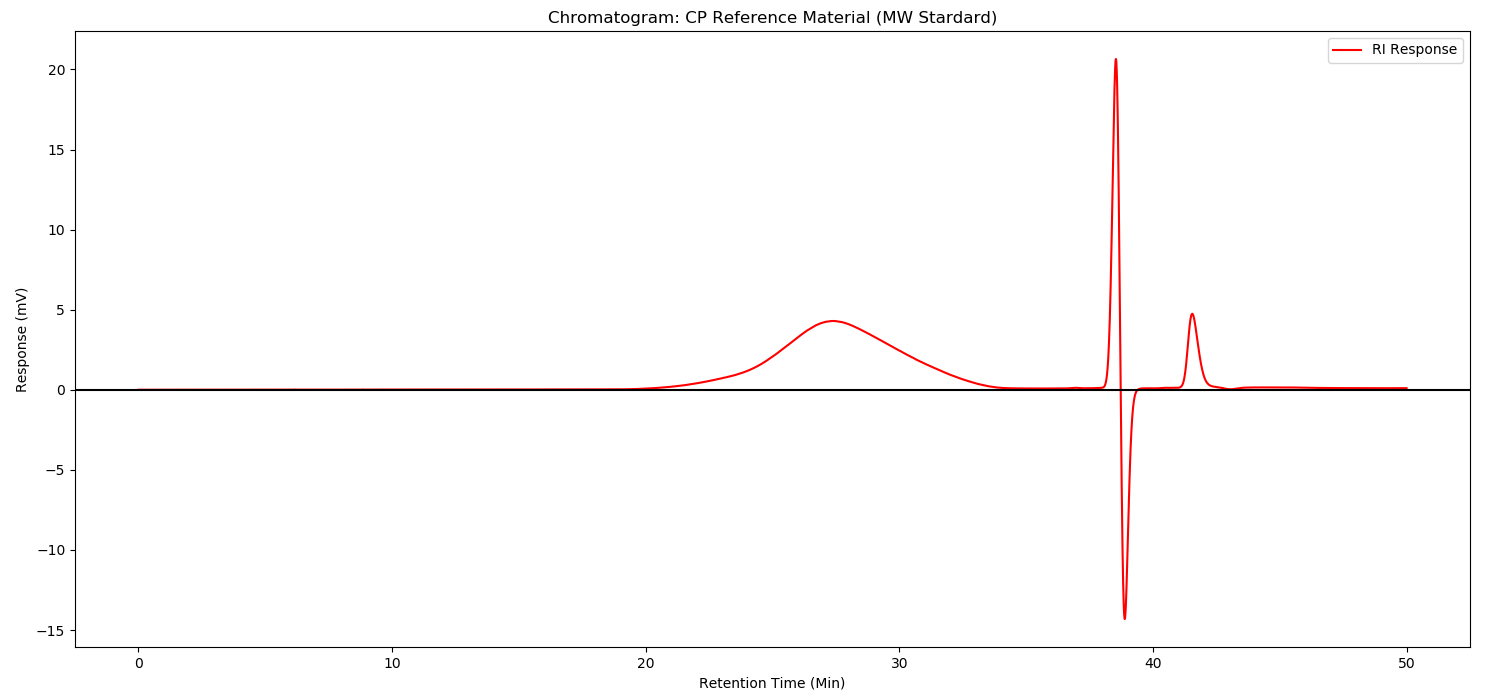
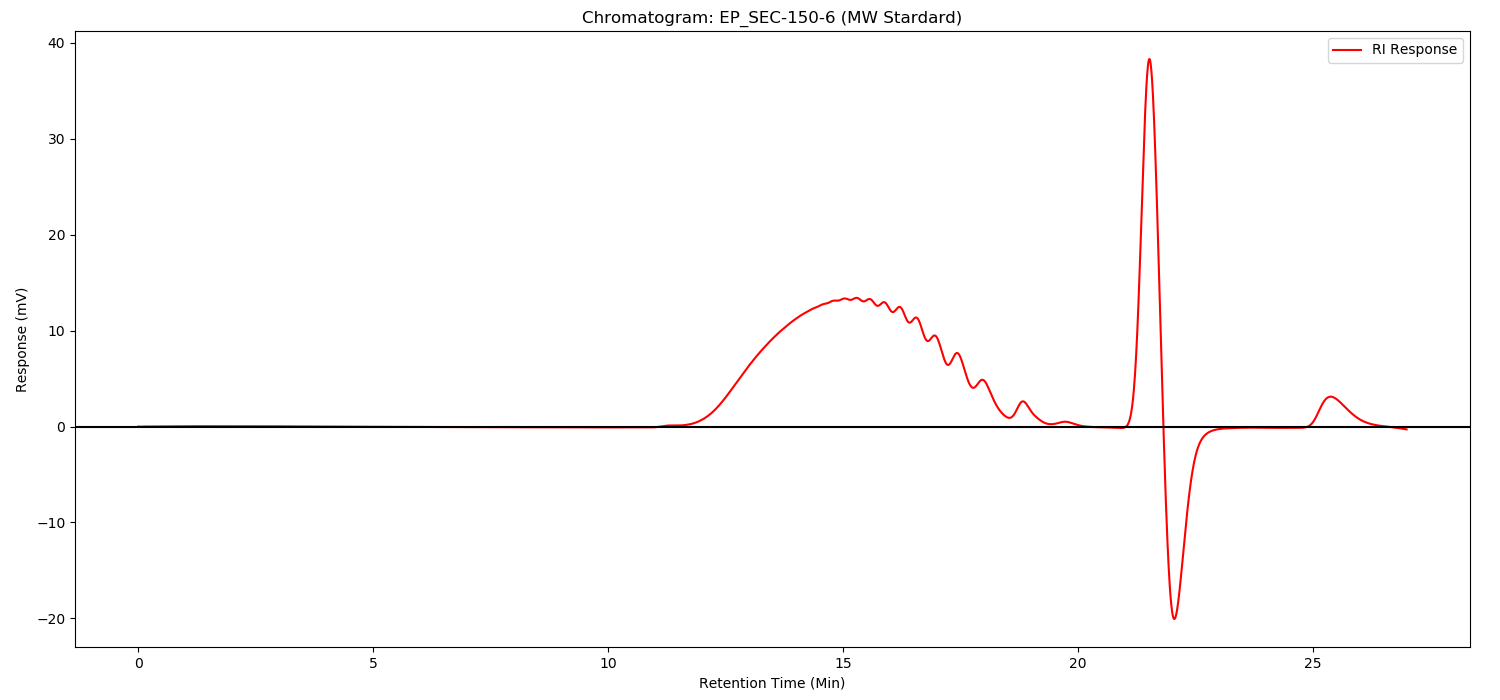
Method:
According to USP about Enoxaparin disaccharide enzymolysis and test method.Before adding Heparinases, you should dilute and use them according to the labeled value of the Heparinases(Heparinase I 10IU/ml, Heparinase II 4IU/ml, Heparinase III 5IU/ml).
Sample groups:
Testing liquid 1:Joint enzymolysis of Heparin sodium solution by Heparinase I,II,III.
Testing liquid 2:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III.
Testing liquid 3:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III.(Heparinase I was replaced by other lots of Heparinase I in stock).
Testing liquid 4:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III.(Heparinase II was replaced by other lots of Heparinase II in stock).
Testing liquid 5:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III.(Heparinase III was replaced by other lots of Heparinase III in stock).
Result:
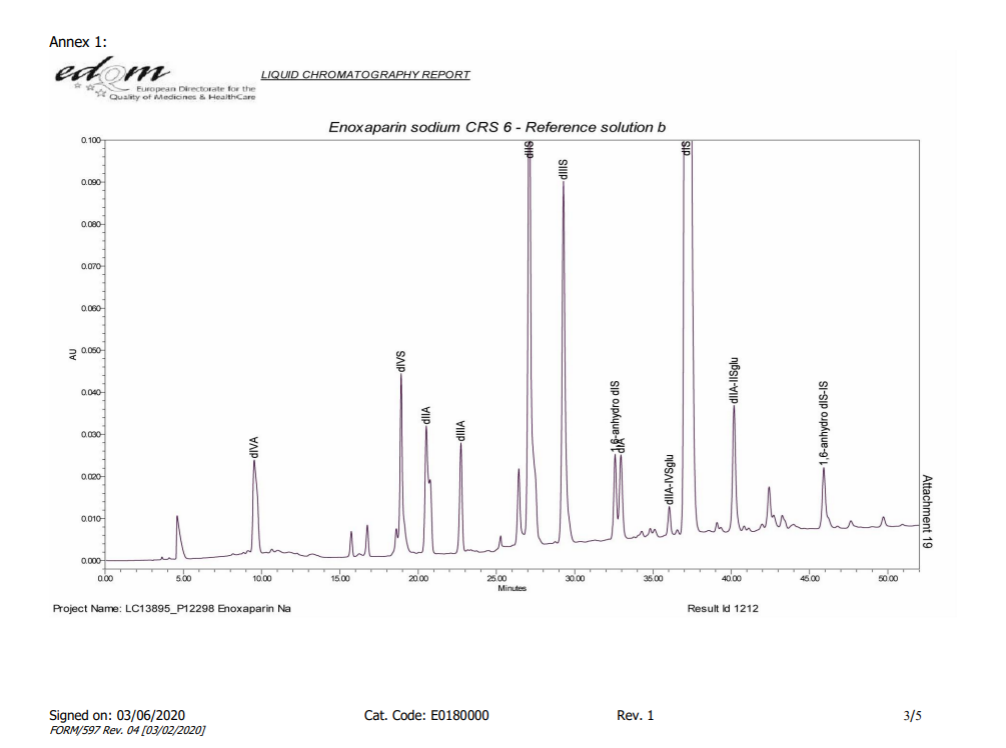
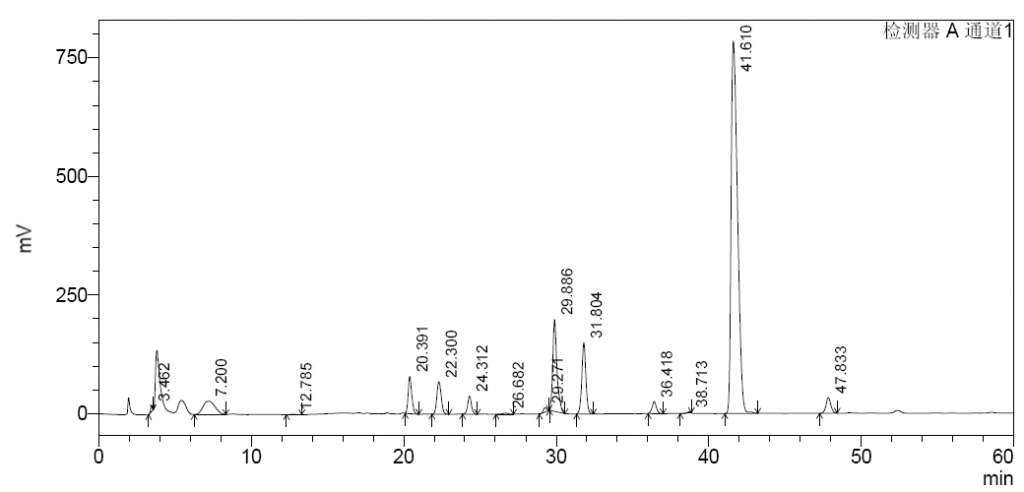
As illustrated in following spectrums.
Analysis:
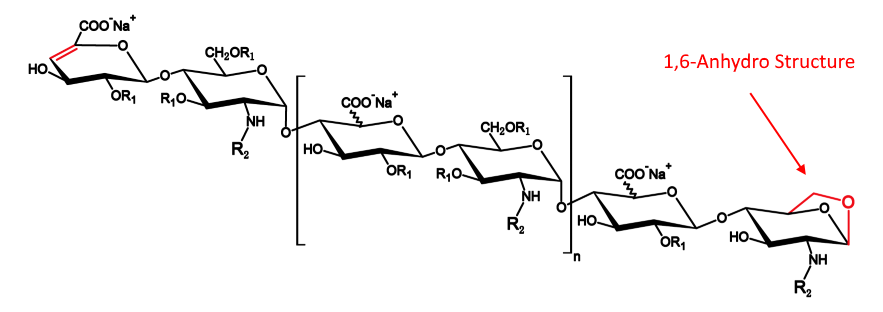
- Because the experimental method is not controlling well,or the efficacy of column is not enough,the separation effect of the separation diagram is not as good as the separation diagram of EDQM,for example 1,6-androIS is inseparable from IA.
- The enzymolysis effect of enoxaparin is sufficient,1,6-androIS at 39.3 retention time,less than 1/2 of the 1,6-androIS and IA mixed peak at 31.1 retention time.
- Enoxaparin enzymolysis by three kinds of Heparinases,Replacing any of the three Heparinases,the peak spectrum changed little.
- In both heparin and enoxaparin,the presence of connective regions can be seen after enzymolysis,the paek at about 19.5 retention time,but they are not high.
- Replacing any of the three Heparinases showed little change in the peak of the junction.It shows that our Heparinases cannot make such a high peak as the connective region in the Changshan Pharmaceutical Company experiment result.Its height is also much higher than the peaks of the standard spectrum.
- Because the instrument operating system has problems,the integration of each peak cannot be realized yet.
Testing liquid 1:Joint enzymolysis of Heparin sodium solution by Heparinase I,II,III
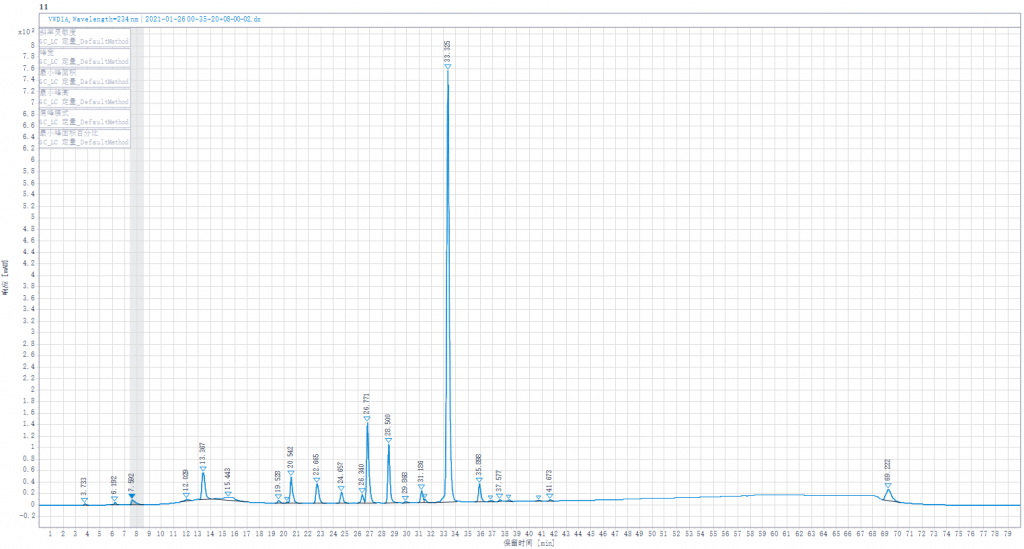
Testing liquid 2:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III
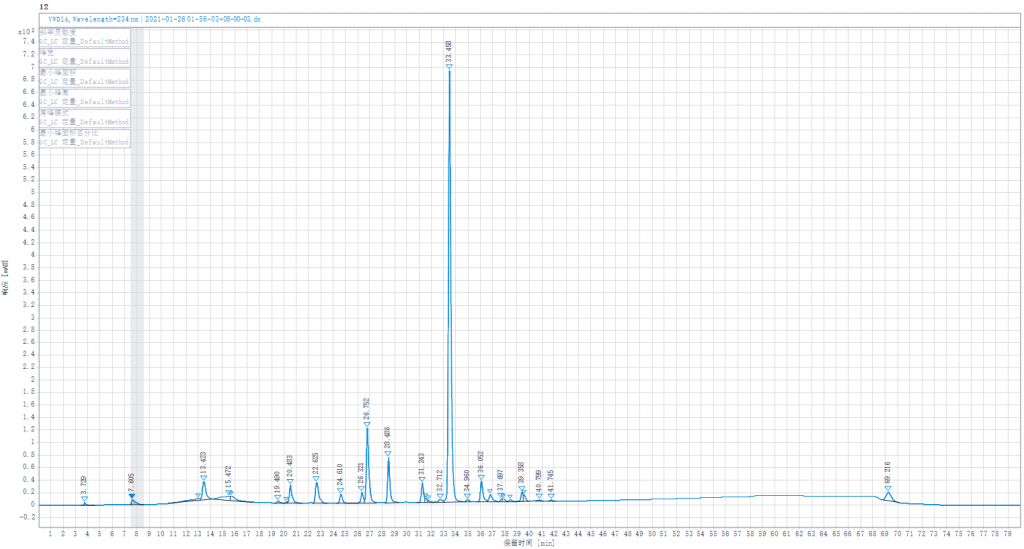
Testing liquid 3:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III,(Heparinase I was replaced by other lots of Heparinase I in stock)
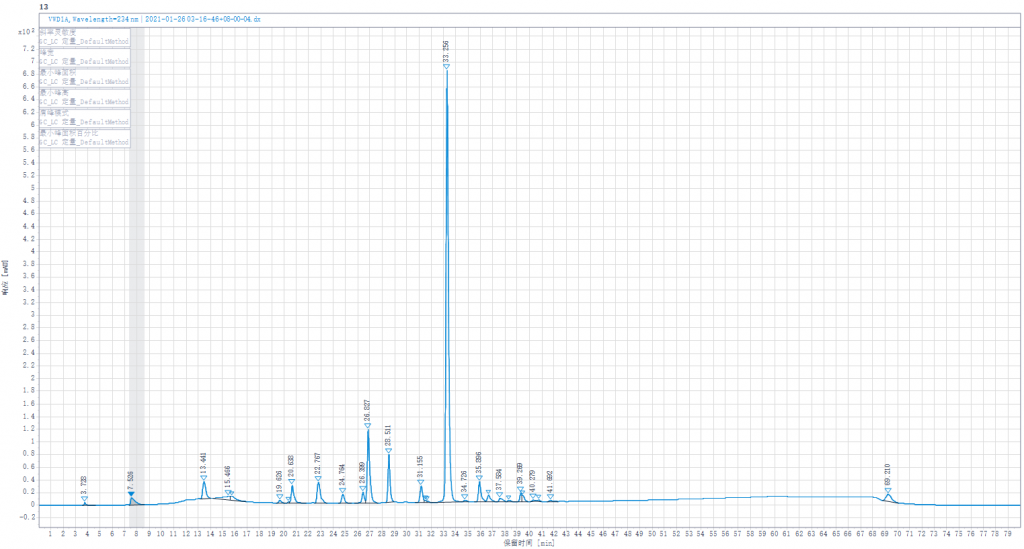
Testing liquid 4:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III,(Heparinase II was replaced by other lots of Heparinase II in stock)
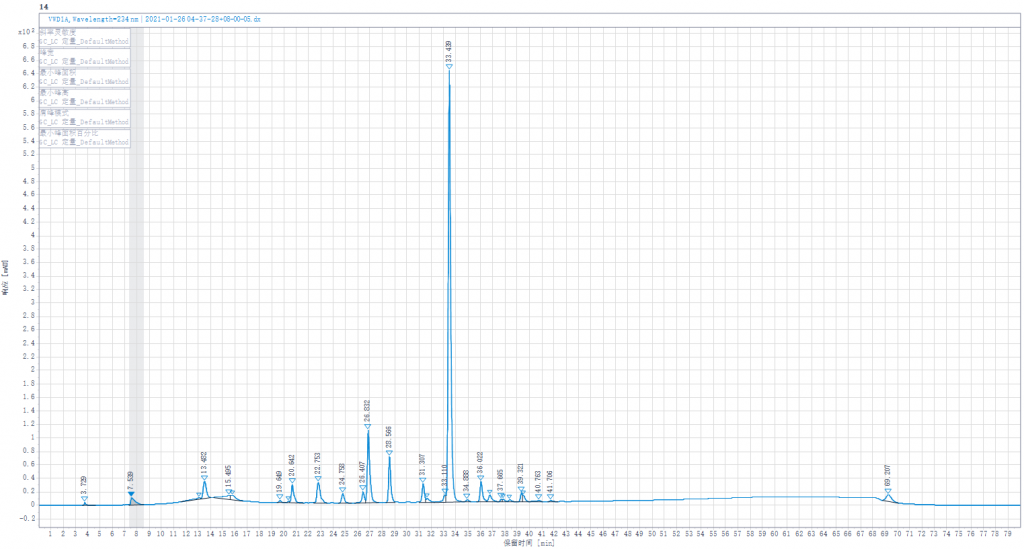
Testing liquid 5:Joint enzymolysis of Enoxaparin sodium solution by Heparinase I,II,III,(Heparinase III was replaced by other lots of Heparinase III in stock)
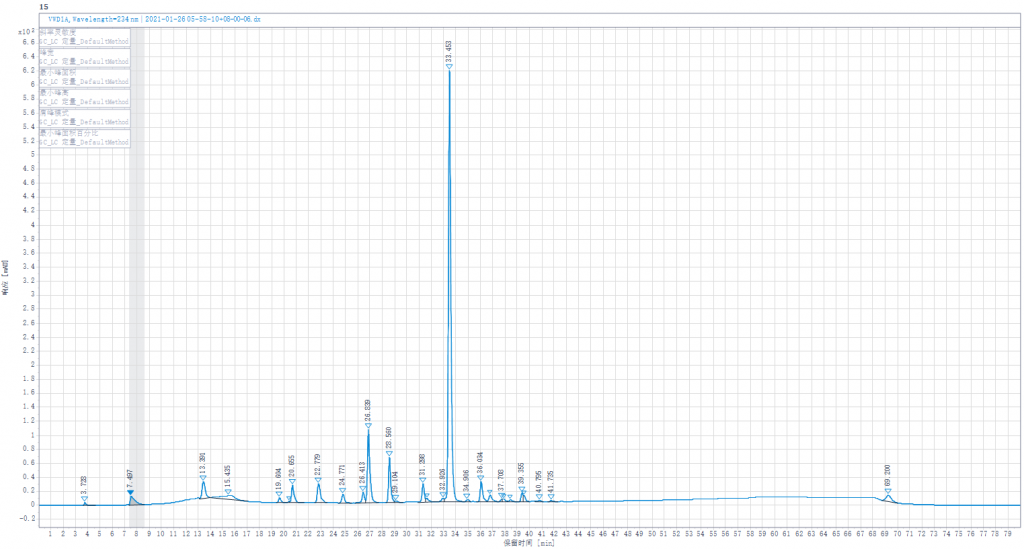
The Spectrum of EDQM