1 Abstract
In this experiment, we followed the United States Pharmacopeia standard for Enoxaparin Sodium released on August 15, 2022, Identification D: Molecular Weight Distribution and Weight-Average Molecular Weight requirements, and used molecular size chromatography to detect the molecular weight and distribution of enoxaparin sodium using two BioCore SEC-150HP chromatographic columns. The results showed that the determination result of the molecular weight calibrant sample (USP Heparin Sodium Molecular Weight Calibrant RS) in this experiment was 4418Da (4370±50Da), and the actual sample test results are shown in Table 6, which meet the pharmacopoeia requirements.。
2 Overview
Enoxaparin Sodium is the sodium salt of a depolymerized heparin. It is obtained by alkaline depolymerization of heparin benzyl ester. The starting material, heparin, is obtained exclusively from porcine intestinal mucosa. Heparin source material used in the manufacture of Enoxaparin Sodium complies with the compendial requirements stated in the Heparin Sodium monograph. Enoxaparin Sodium consists of a complex set of oligosaccharides that have not yet been completely characterized. The majority of the components have a 4-enopyranose uronate structure at the nonreducing end of their chain. About 20% of the materials contain a 1,6-anhydro derivative on the reducing end of the chain, the range being between 15% and 25%. The weight-average molecular weight of Enoxaparin Sodium is 4500 Da, the range being between 3800 and 5000 Da; about 16% have a molecular weight of less than 2000 Da, the range being between 12.0% and 20.0%; about 74% have a molecular weight between 2000 and 8000 Da, the range being between 68.0% and 82.0%. NMT 18.0% have a molecular weight higher than 8000 Da. When prepared as a solution, the solution is analyzed for clarity and degree of color using a validated method. The degree of sulfation is NLT 1.8 per disaccharide unit.
Calculations: Compute the data, using the same GPC software; determine the weight-average molecular weight, Mw, for each of the duplicate chromatograms of the Standard solution and the Sample solution; and take the average for each solution. Correct the mean value of Mw to the nearest 50. The Chromatographic system is suiTable if Mw for USP Enoxaparin Sodium RS is within 150 Da of the labeled Mw value. The Mw for the Sample solution is between 3800 and 5000 Da. Using the same software, determine for each of the duplicate Sample solution chromatograms the percentage of Enoxaparin Sodium chains with molecular weights lower than 2000 Da, M2000, the percentage of Enoxaparin Sodium chains with molecular weights in the range 2000–8000 Da, M2000-8000, and the percentage of Enoxaparin Sodium chains with molecular weights greater than 8000 Da, M8000. Average the duplicate values, and express to the nearest 0.5%.
Acceptance criteria: M2000 is between 12.0% and 20.0%, M2000-8000 is between 68.0% and 82.0%, and M8000 is NMT 18.0%.
3 Experimental Method
Standard solution: 10 mg/mL of USP Enoxaparin Sodium RS in Mobile phase.
Sample solution: 10 mg/mL of Enoxaparin Sodium in Mobile phase.
Analysis: Reconstitute 1 vial each of USP Enoxaparin Sodium Molecular Weight Calibrant A RS and USP Enoxaparin Sodium Molecular Weight Calibrant B RS in 1 mL of Mobile phase. Separately inject 20 µL of USP Enoxaparin Sodium Molecular Weight Calibrant A RS and USP Enoxaparin Sodium Molecular Weight Calibrant B RS, record the chromatograms, and measure the retention times. Inject in duplicate 20 µL each of the Standard solution and the Sample solution, and record the chromatograms for a length of time to ensure complete elution, including salt and solvent peaks. Calculate the total peak areas under each of the Standard solution and Sample solution chromatograms, excluding salt and solvent peaks at the end.
Mobile phase: 0.5 M lithium nitrate solution
Flow rate: 0.6 ml/min ± 0.1%
Column temperature: 30°C
Detector: Differential Refractive Index (DRI) detector
Injection volume: 20 μl
Column: Two BioCore SEC-150HP columns in series, 5μm, 7.8×300mm
4 Experimental Results
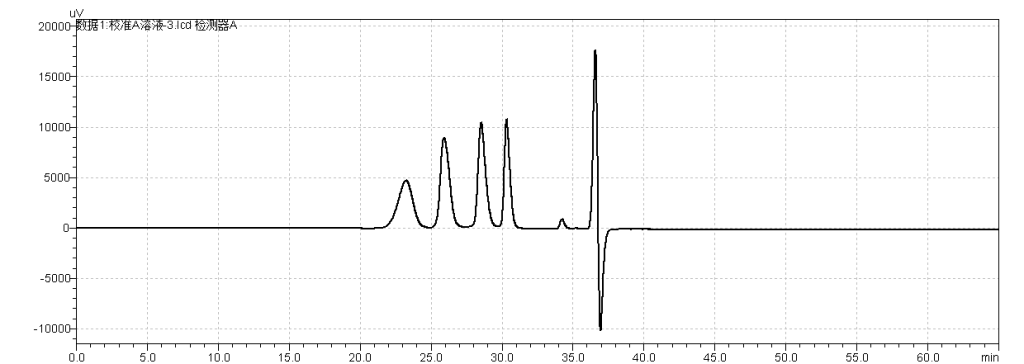
fig.22. Chromatogram of USP Enoxaparin Sodium Molecular Weight Calibrant A RS
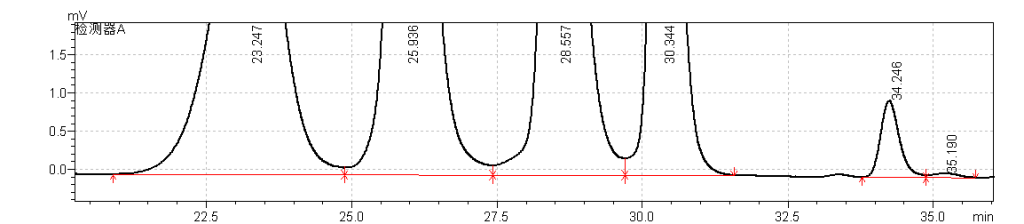
fig.23. Magnified Chromatogram of USP Enoxaparin Sodium Molecular Weight Calibrant A RS
fig.24. Chromatogram of USP Enoxaparin Sodium Molecular Weight Calibrant B RS
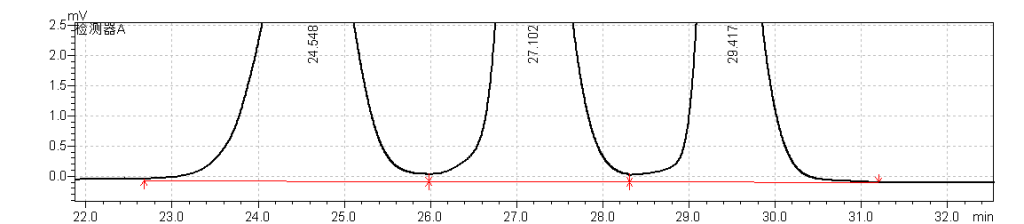
fig.25 Magnified Chromatogram of USP Enoxaparin Sodium Molecular Weight Calibrant B RS
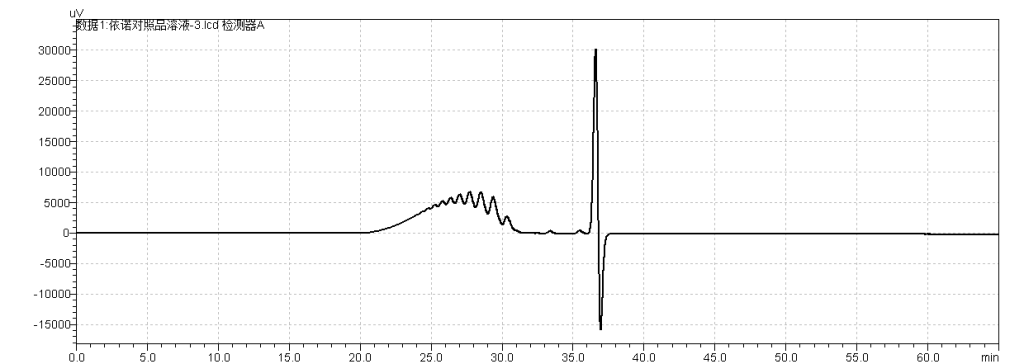
fig.26. Chromatogram of USP Enoxaparin Sodium RS
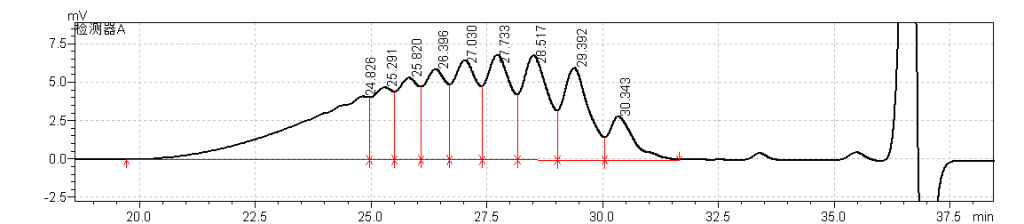
fig.27.Magnified Chromatogram of USP Enoxaparin Sodium RS
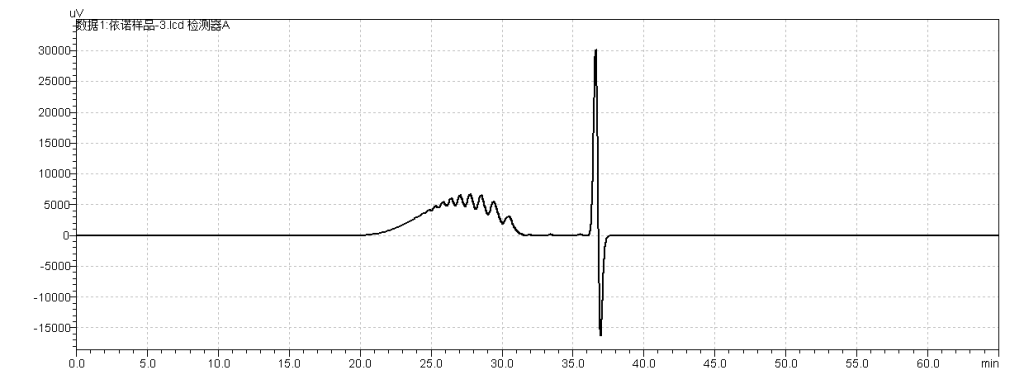
fig.28. Chromatogram of Sample
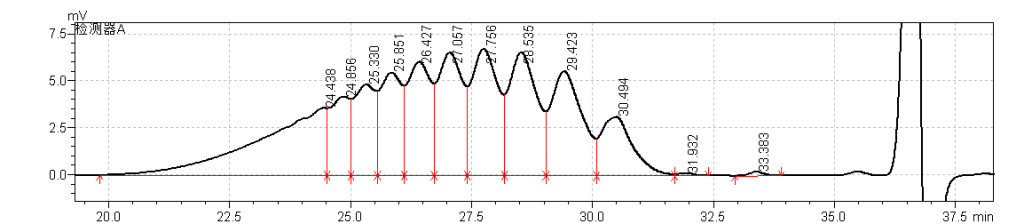
fig.29. Magnified Chromatogram 1 of Sample
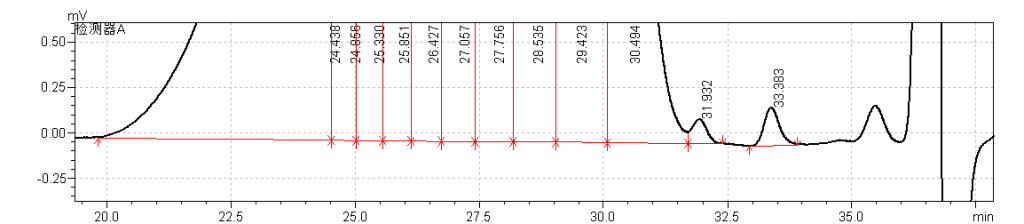
fig.30. Magnified Chromatogram 2 of Sample
Table. Experimental Results of Enoxaparin sodium sample
| Molecular Weight and Molecular Weight Distribution | Mw between3800-5000Da。 | 4446Da |
| M2000 between 12.0% and 20.0%。 | 19.1% | |
| M2000-8000 between 68.0% and 82.0% | 70.8% | |
| M8000 NMT 18.0% | 10.1% |

