1 Abstract
In this experiment, we followed the European Pharmacopoeia (11.2) Heparins, low-molecular-mass standard, Identification C: Size-exclusion chromatography requirements, and used molecular size chromatography to detect the molecular weight and distribution of enoxaparin sodium using a BioCore SEC-150HP chromatographic column. The results showed that the determination result of Enoxaparin sodium CRS in this experiment was 4476Da (4367±150Da), and the actual sample test results are shown in Table 5, which meet the pharmacopoeia requirements.
2 Overview
Salts of sulfated glycosaminoglycans having a mass-average relative molecular mass less than 8000 and for which at least 60 per cent of the total mass has a relative molecular mass less than 8000. Low-molecular-mass heparins display different chemical structures at the reducing or the non-reducing end of the polysaccharide chains.
Low-molecular-mass heparins are obtained by fractionation or depolymerisation of heparin of natural origin that complies with the monograph Heparin sodium (0333) or Heparin calcium (0332), whichever is appropriate, unless otherwise justified and authorised. For each type of low-molecular-mass heparin the batch-to-batch consistency is ensured by demonstrating, for example, that the mass-average relative molecular mass and the mass percentage within defined relative molecular-mass ranges lower than 8000 are not less than 75 per cent and not more than 125 per cent of the mean value stated as type specification.
Enoxaparin sodium is the sodium salt of a low-molecular-mass heparin that is obtained by alkaline depolymerisation of the benzyl ester derivative of heparin from porcine intestinal mucosa. Enoxaparin consists of a complex set of oligosaccharides that have not yet been completely characterised. Based on current knowledge, the majority of the components have a 4-enopyranose uronate structure at the non-reducing end of their chain. 15 per cent to 25 per cent of the components have a 1,6-anhydro structure at the reducing end of their chain.
Enoxaparin sodium complies with the monograph Low-molecular-mass heparins (0828) with the modifications and additional requirements below.
Where no individual monograph exists for the low-molecular-mass heparin to be examined, the mass-average relative molecular mass is not greater than 8000 and at least 60 per cent of the total mass has a relative molecular mass lower than 8000. In addition, the molecular mass parameters (mass-average molecular mass and mass percentages of chains comprised between specified values) correspond to those of the manufacturer’s reference preparation.
3 Experimental Method
Test solution. Dissolve 20 mg of the substance to be examined in 2 mL of the mobile phase.
Reference solution. Dissolve 20 mg of heparin low-molecular-mass for calibration CRS in 2 mL of the mobile phase.
Mobile phase: 7.7 g/L ammonium acetate solution
Flow rate: 0.5 mL/min
Column temperature: 30°C
Detector: Differential Refractive Index Detector
Injection volume: 25 μL
Column: BioCore SEC-150HP, 5μm, 7.8×300mm.
4 Experimental Results
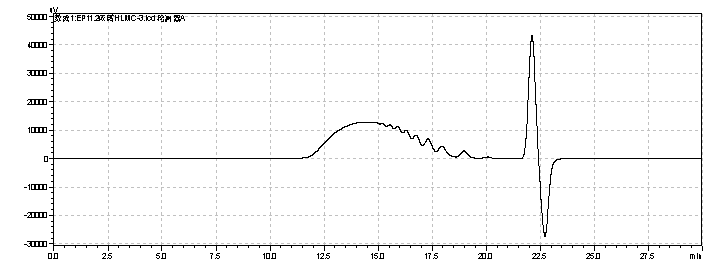
fig.16. Chromatogram of heparin Low-Molecular-Mass for calibration CRS
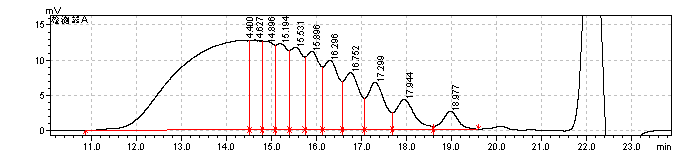
fig.17. Magnified Chromatogram of heparin Low-Molecular-Mass for calibration CRS
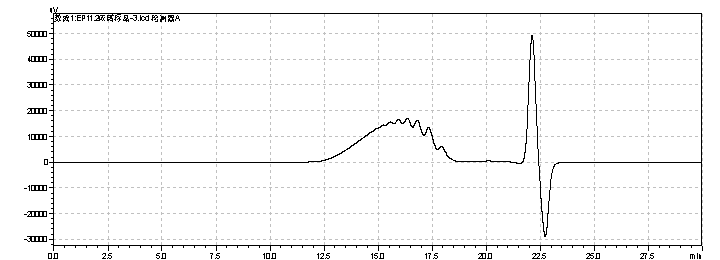
fig.18. Chromatogram of Enoxaparin sodium CRS
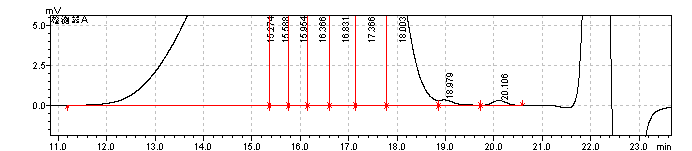
fig.19. Magnified Chromatogram of Enoxaparin sodium CRS
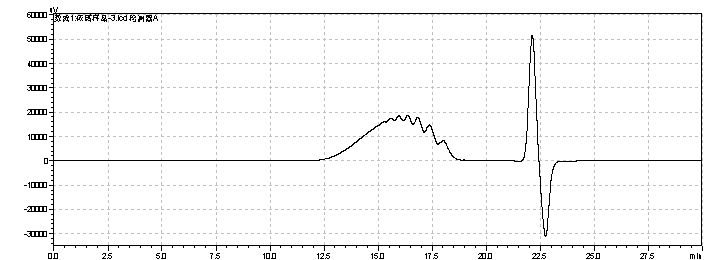
fig.20. Chromatogram of Sample
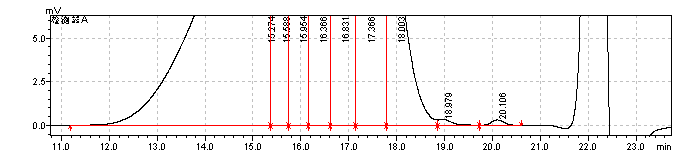
fig.21. Magnified Chromatogram of Sample
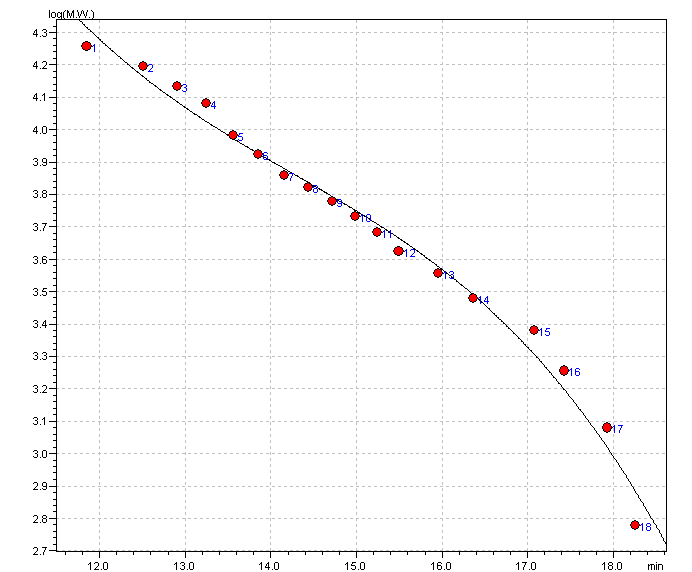
third-order polynomial equation : aX^3+bX^2+cX+d (X=x-T.LIMIT)
a = -0.005900979 b = 0.2528999 c = -3.765381
d = 23.24280
T.LIMIT = 0 min
R^2 = 0.9858275 R = 0.9928885 Dispersion of Calibration Points = 0.04586096
Table Experimental Results of Enoxaparin sodium sample
| Molecular Weight and Molecular Weight Distribution | Mw between 3800-5000Da。 | 4284Da |
| M<8000 between 75%-125%。 | 91% |

