1 Abstract
In this experiment, according to the Molecular Weight Determinations requirement in Identification D of the United States Pharmacopeia Heparin Sodium standard released on August 23, 2022, the molecular weight and molecular weight distribution of heparin sodium were detected by size exclusion chromatography using BioCore SEC-500HP and BioCore SEC-300HP columns. The results showed that the USP Heparin Sodium Identification RS measurement in this experiment was 15774Da (16000±500Da), and the actual sample test results are shown in Table 3, which are in accordance with the requirements of the Pharmacopeia.。
2 Overview
Heparin Sodium is the sodium salt of sulfated glycosaminoglycans present as a mixture of heterogeneous molecules varying in molecular weights that retains a combination of activities against different factors of the blood clotting cascade. It is present in mammalian tissues and is usually obtained from the intestinal mucosa or other suiTable tissues of domestic mammals used for food by humans. The sourcing of heparin material must be specified in compliance with applicable regulatory requirements. The manufacturing process should be validated to demonstrate clearance and inactivation of relevant infectious and adventitious agents (e.g., viruses, TSE agents). See Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin á1050ñ for general guidance on viral safety evaluation. The heparin manufacturing process should also be validated to demonstrate clearance of lipids. It is composed of polymers of alternating derivatives of α-D-glucosamido (N-sulfated, O-sulfated, or N-acetylated) and O-sulfated uronic acid (α-L-iduronic acid or β-D-glucuronic acid). The component activities of the mixture are in ratios corresponding to those shown by USP Heparin Sodium for Assays RS.
Suitability requirements
Weight-average molecular weight ( M w): Take the mean of the calculated M w from the duplicate injections of the System suitability solution, and round to the nearest 100 Da. The chromatographic system is suiTable if the M w of the System suitability sample is within 500 Da of the labeled value as stated in the USP Certificate for USP Heparin Sodium Identification RS.
Peak molecular weights ( M p): The peak molecular weights (M p) of the duplicate injections of the System suitability solution do not differ by more than 5% of the upper value.
Resolution: There is baseline resolution between the heparin and salt peaks.
Calibration curve: The linear regression coefficient of the calibration curve fitted to the Broad Standard Table values must be NLT 0.990, using a third-order polynomial equation.
Acceptance criteria: M 24000 is NMT 20%, M w is between 15,000 Da and 19,000 Da, and the ratio of M 8000–16000 to M 16000–24000 is NLT 1.0.
3 Experimental Method
Calibration solution: Prepare by dissolving 10 mg of the USP Heparin Sodium Molecular Weight Calibrant RS in 2 mL of Mobile phase.
System suitability solution: 5 mg/mL of USP Heparin Sodium Identification RS in Mobile phase.
Sample solution: Dissolve about 10 mg of Heparin Sodium sample in 2 mL of Mobile phase.
Mobile phase: 0.1 mol/L acetic acid ammonium solution
Flow rate: 0.6 ml/min ±0.1%
Column temperature: 30℃
Detector: Differential Refractive Index Detector
Injection volume: 20 μL
Chromatographic column: BioCore SEC-500HP, 5μm, 7.8×300mm, connected in series with BioCore SEC-300HP, 5μm, 7.8×300mm
4 Experimental Results
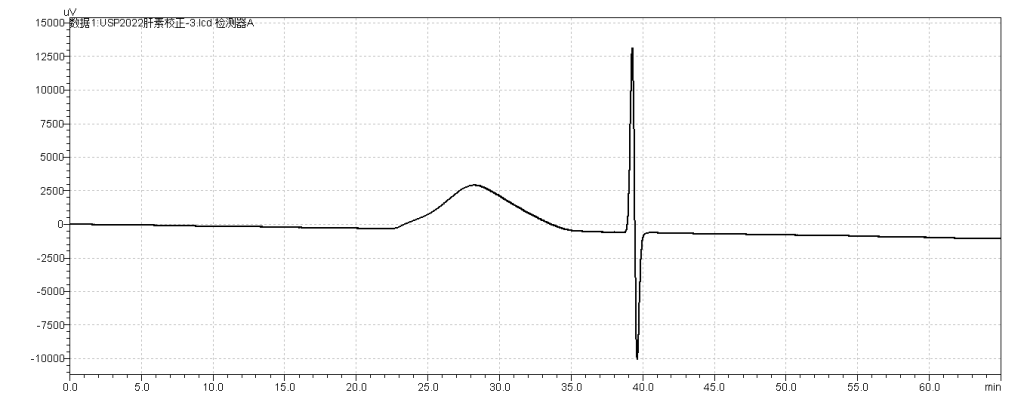
fig.1.Chromatogram of Reference strands
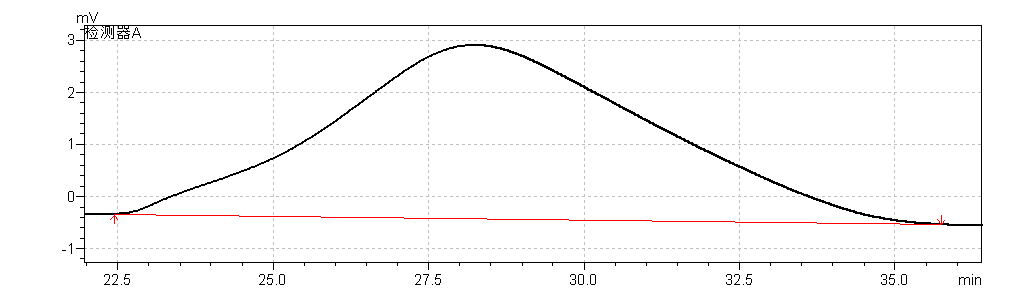
fig.2. Magnified Chromatogram of Reference standards
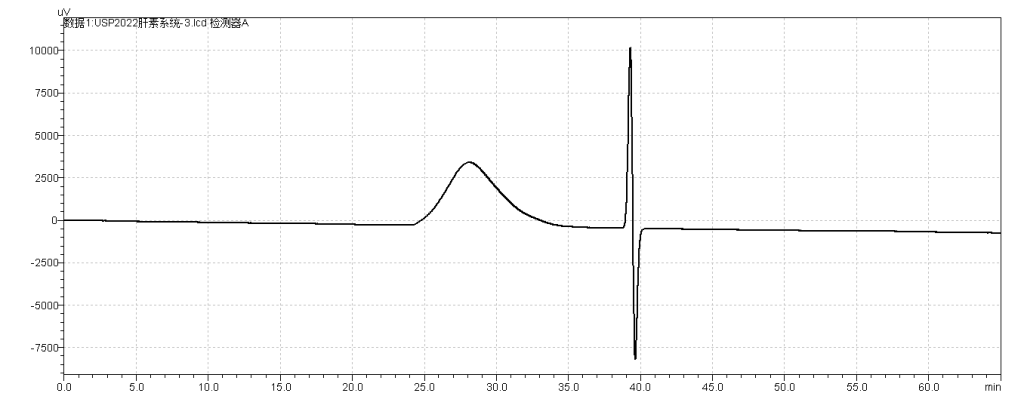
fig.3. Chromatogram of Suitability reference material
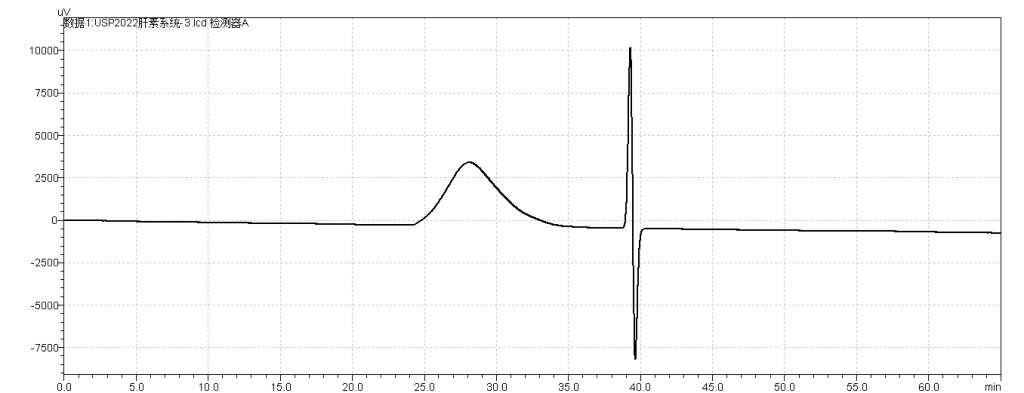
fig.4. Chromatogram of Sample
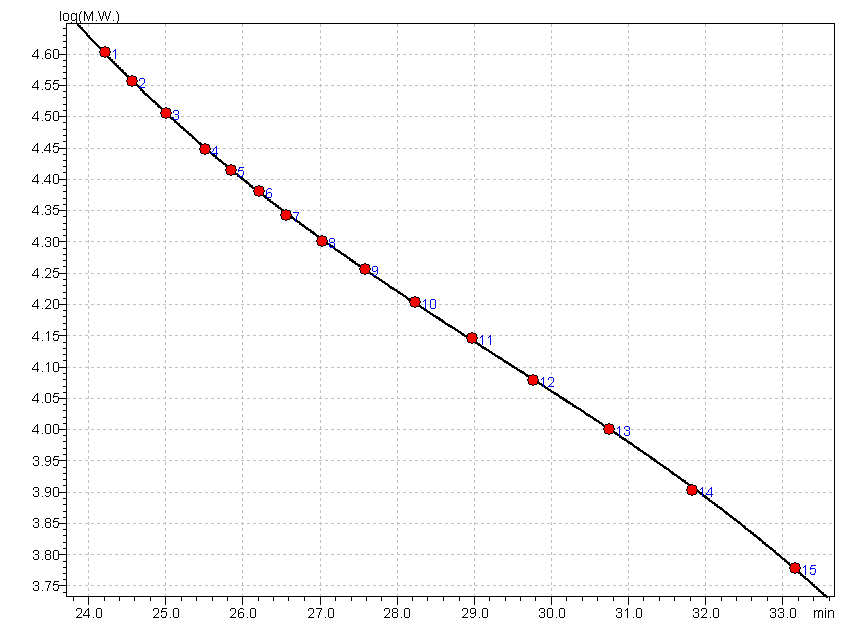
third-order polynomial equation : aX^3+bX^2+cX+d (X=x-T.LIMIT)
a = -0.0006215789 b = 0.05468368 c = -1.682499
d = 210360
T.LIMIT = 0 min
R^2 = 0.9999109 R = 0.9999555 Dispersion of Calibration Points = 0.002210642
Table . Experimental Results of sample
| Molecular Weight and Molecular Weight Distribution | M w is between 15,000 Da and 19,000 Da。 | 15473Da |
| he ratio of Mw8000-16000 to Mw16000-24000 is NLT 1.0。 | 1.7 |

