The percentage of 1,6-anhydro species is a unique characteristics of enoxaparin sodium, one of low-molecular-weight heparins (LWMHs) that have been widely used as anticoagulants.
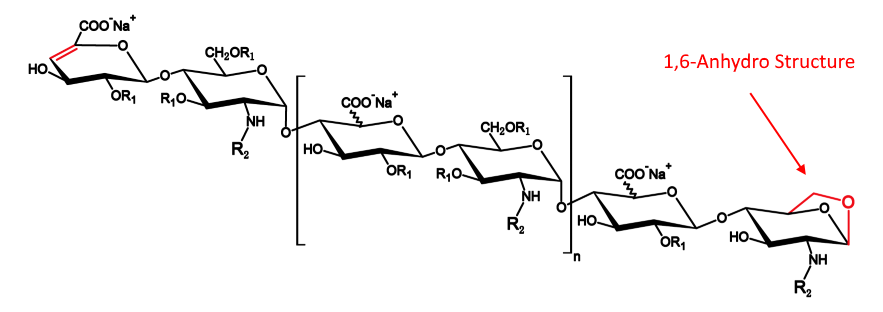
How to determine this structure?
Procedures to determine levels of 1,6-anhydro structure are listed in pharmacopeias like EP <1097> and USP <207>.
To determine the percentage of 1,6-anhydro species, enoxaparin sodium samples are first degraded via a combination of heparinases I, II and III, followed by reduction via sodium borohydride. The degraded and reduced sample solutions are injected into a HPLC system for analysis.
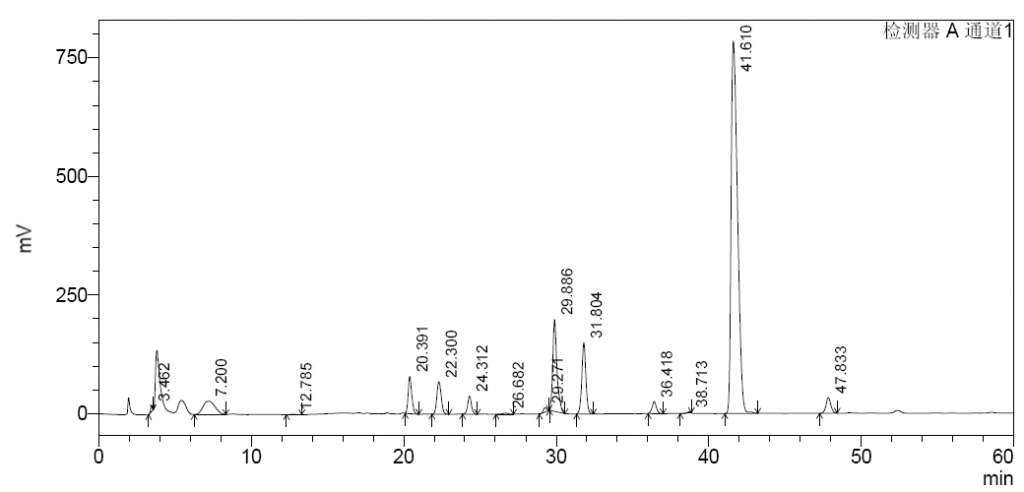
Successful analysis of this unique characteristics in enoxaparin sodium heavily depends on the choice of right HPLC columns and heparinases.
Why choose Asnail?
For analysis of 1,6-anhydro species in enoxaparin sodium, Asnail offers heparinases, heparin-derived unsaturated disaccharides, and SAX columns.
Heparinases are the most important and expensive reagents used in QA of enoxaparin sodium. Asnail’s native heparinases feature great stability and purity. By now, Asnail’s heparinases have served many customers whose procedures are in compliance with EN and USP.

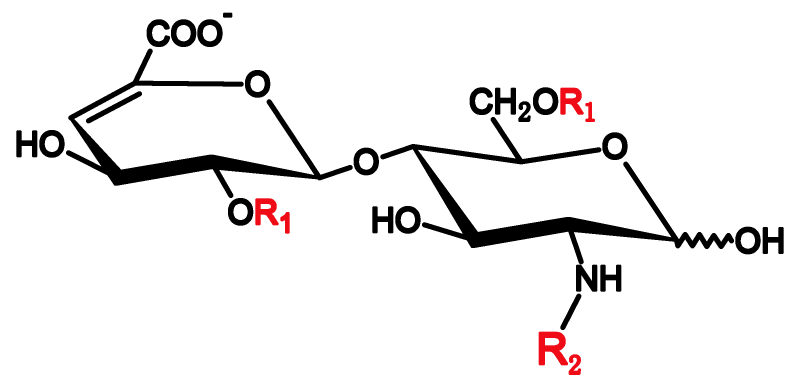
To identify peaks in chromatograms, Asnail provides heparin unsaturated disaccharides as reference materials which are prepared by degrading heparin and/or heparan sulfate via heparinases.
For HPLC columns, Asnail has tested and selected the Ultisil XB-SAX analytical column, which has proved to provide better performance than previously available columns. This column is co-developed with Welchmat, a long-time parter of Asnail.

Product links
Heparinases from Flavobacterium heparinum

